What Is Chemical Characterisation of Biomaterials and Medical Devices?
At Applus+ Laboratories, we offer comprehensive chemical characterisation of biomaterials and medical devices involving routine analysis of raw materials and final products, contamination identification, change control monitoring, reverse engineering, and audits. These processes help us identify residual and contamination during the manufacturing process and, depending on the results, can include further work and development with our experts.
What Chemical Characterisation of Biomaterials and Medical Devices Do We Offer?

Chemical Analysis
- Polymer molecular weight
- Polymer nature confirmation
- Endocrin disruptor (Bisphenol A, phthalates...)
- Chemical dosage
- Chemical change
- Chemical analysis of surface (MEB/EDS)

Chromatography & Spectrometry
- Impact of process
- Additives and adjuvants
- Residual solvent
- Residual monomers
- Material purity
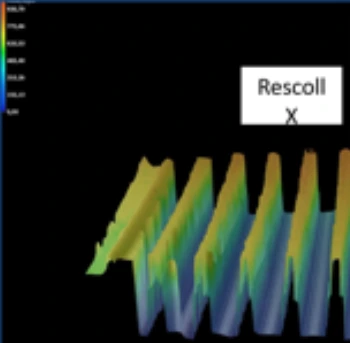
Surface Analysis
- Surface morphology
- Surface topography
- Roughness assessment
- Particle size distribution
- Dimensional measurement
- Wettability and hydrophilicity
- Adhesion tests & tribology

Thermal Analysis
- Glass transition, melting point
- Use temperature range
- Crystallinity
- Water content
- Ash content
- Modulus under dynamic stress
Spectrometry and Chromatography Characterisation for Quality Control
Chemical characterisation consists of identifying the presence of unexpected and unwanted compounds as well as verifying the quality of a material that might have developed during material reception, manufacturing, cleaning, or sterilisation. At Applus+ Laboratories, we offer the following routine analyses:
- Polymer chemical identification by FTIR
- Gas and liquid chromatography (HPLC, LC-MS, GC-MS Pyro-GC-MS): We identify and quantify the presence of SVOCs, VOCs, and NVOCs, such as cleaning agents, additives, fillers, and polymers. The presence of endocrine disruptor, residual solvent or monomed, chemical chemical of concern, PFAS and other additives that could bring safety issue
- Elemental Analysis and Metal Traces: We utilise ICP-OES spectrometry to measure and remove elemental impurities in medical devices.
- Polymer molecular weight by SEC: We perform size exclusion chromatography to provide the molecular weight distribution of polymers, which influences their mechanical and physical properties, affecting the device's performance and durability.
Physico-Chemical and Material Characterisation
Material characterisation involves identifying any changes in the intrinsic properties of materials associated with manufacturing, cleaning, and/or sterilisation. These analyses can also be performed to qualify the material after manufacturing. We offer the following analyses:
- Water Content: Knowing the amount of water is important to evaluate the initial state of the material and a potential change in properties.
- Viscosity measurement using Brookfield, rheometer or gel timer apparatus
- Residual Hydrogen Peroxide
We employ spectrophotometry and titration to ensure safe levels of residual hydrogen peroxide. - Chemical dosage
Surface Analysis
These analyses are coupled with a comprehensive assessment of the surface state, including topography, wettability, roughness, and wear resistance. These tests allow verification of the quality of the interface and adhesion between coatings and medical devices (to be linked with material and coating sheet).
- Particle Size Characterisation: We use diffraction, laser granulometry, and high resolution microscopy to identify particle sizes to control the release rate of coatings on joint implants and the presence of contaminants in medical devices.
- SEM Microscopy with Elemental Analysis: We employ Scanning Electron Microscopy (SEM) which provides high resolution images of the surface morphology of medical device coatings and presence of particles on the surface.This is coupled with elemental analysis that provides the element composition of the structure.
Thermal Analysis and Thermomechanical Properties with DSC and DMA
Differential scanning calorimetry (DSC) measures the glass transition temperature and melting properties of polymers, which can be useful for controlling polymer batches, ensuring that sterilisation does not affect the crystallinity of the medical device surface, and controlling the polymerisation process as well as measuring the extent of cure of thermoset resins.
Dynamic Mechanical Analysis (DMA) evaluates mechanical stress under cyclic conditions or over a range of temperature to bring dynamic mechanical assessment and visco elastic behavior of a material.
What Are the Benefits of Chemical Characterisation of Biomaterials and Medical Devices?
Material and chemical characterisation, along with being an essential part of our batch testing service, also has a lot of advantages for both ensuring patient safety and for maintaining compliance to medical device related standards. These benefits include this following:
- Performance and Reliability: Assessing the properties of your product can ensure that the materials used, and the design are reliable and will perform as it should in the range of different stress scenarios it will undergo.
- Patient Safety: Undergoing material and chemical characterisation reduces the adverse effects that patients might experience from the materials coming into contact with bodily fluids or tissues. It greatly reduces the risk of toxicity from harmful materials to maintain the product as safe as possible.
- Regulatory Compliance and Market Access: Material and chemical characterisation ensures that you follow the stringent standards put in place for medical devices. This regulatory compliance allows you to boost consumer trust knowing that your product has been fully tested.
- Risk Mitigation and Management: Material and chemical characterisation can assess potential risks and give advice on design changes in order to properly mitigate these risks. This has a great benefit to patient safety as well as consumer confidence.
- Market Access: Complying with the relevant standards when it comes to medical devices means boosting your market access to a global scale. With the right certifications, you’re more likely to operate in more countries.
Why Choose Applus+ Laboratories for Chemical Characterisation?
Choosing Applus+ Laboratories for your material and chemical characterisation means partnering with a leader in medical device testing solutions. We provide high-quality, ASTM and ISO-compliant testing services that ensure the accuracy and reliability of your medical devices. We offer:
With a presence in multiple countries, we can deliver our testing services to customers around the world, ensuring you have access to the best medical device testing no matter where you are.
Let Applus+ Laboratories be your trusted partner for all your medical device testing needs. We can support your projects with our high-quality services and expert guidance.