At Applus+ Laboratories we can provide you with our excellent medical device testing services, which help you improve the development and the compliance assessment to regulatory standards of your medical devices and their biocompatibility, while also checking the safety of your products. Biocompatibility testing is included with our other batch release testing services:
- Cleaning validation
- Packaging Validation
- Stability Validation
- Material and Chemical Characterisation
What Is Biocompatibility Testing?
Biocompatibility testing is a process that evaluates the compatibility of a medical device or a biomaterial with biological systems to ensure it does not produce adverse effects when in contact with the body and confirms the device will be safe for the patient.
The chemical characterisation is one of the pillars of the biological evaluation of a medical device. First of all the objective of this characterisation is to completely understand the composition and the potential of a medical device to release anything in the body. A critical review of these results obtained could limit the obligation to perform in vivo evaluation.
The chemical characterisation includes several part of the standard ISO 10993:
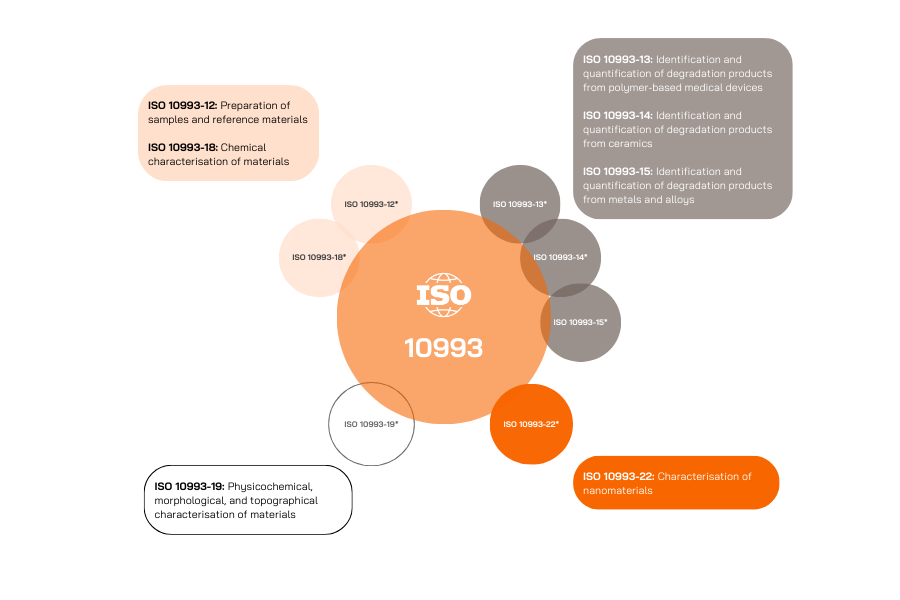
Guidelines to perform the biocompatibility assessment can be found in ISO 10993-1 and provides the required tests to be performed according to the type of device, the contact duration and the nature of contact with the body.
Special focus is required on the material potential to release compounds (associated to the manufacturing process, or the material degradation at its initial stage and after aging to validate the expiry date.
What Physico-Chemical Characterisation Validation Services Do We Offer?
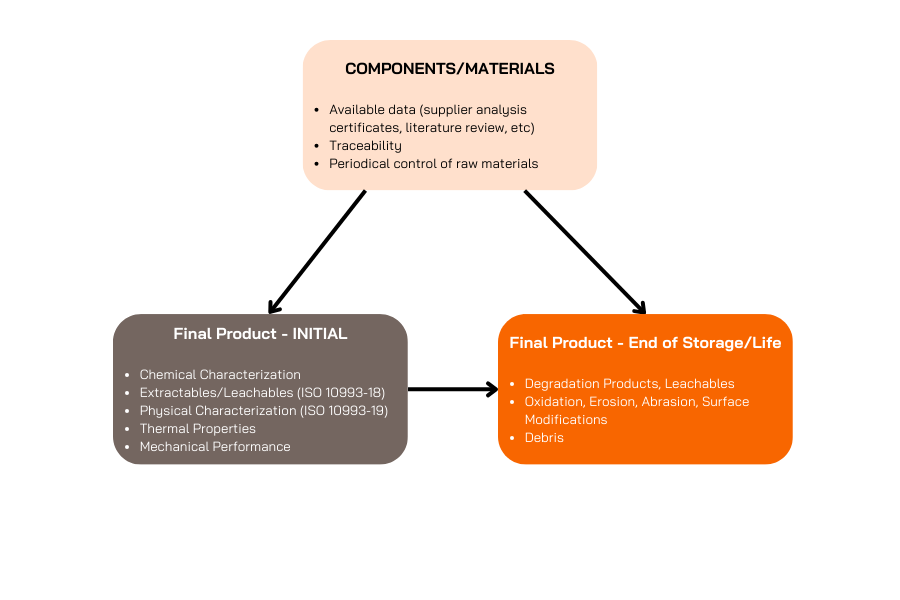
Characterising the material at its initial stage is important to determine the level of risk and releasing chemical compounds or uncontrolled products that could have an impact on the overall safety of the devices. This characterisation is the very first step in the elaboration of the biological evaluation strategy and requirement.
The determination of chemical characterisation after aging and the interaction of the medical device with its packaging is equally important to establish the shelf life and the determination of peremption date.
Physicochemical Analyses According to ISO 10993-12 and ISO10993 -18
This initial analysis is focused on studying the physical and chemical properties of the materials used in medical devices.
All specimens of medical devices are extracted according to ISO 10993+-12 requirements (depending on the nature of contact, duration, targeted market and chemical nature of the device).
The standard ISO 10993-18 provides guidance on identifying and quantifying chemical substances that could potentially be released from the medical device material, extractable and leachable substances, the chemical composition of the material and its chemical structure.
- Analysis of volatile compounds (VOC) such as residual solvent (terpenoid) and alcohols using Headspace gas chromatography (HS-GC-MS)
- Analysis of semi- volatile compounds (SVOC) such as residual monomer and low weight additives (alcanes, alcohols, ester,siloxanes…) using gas chromatography (GC-MS)
- Analysis of non-volatile compounds (NVOC) such as manufacturing and materials additives (high weight additive) using liquid chromatography (LC-MS)
- Analysis of mineral and inorganic compounds using ICP-OES and ICP-MS
- Analysis of ionic compounds (phosphate, chlorate, nitrate, bromide,…) using ionic chromatography (CI)
H3: Physicochemical, Morphological and Topographical Characterisation of Materials According to ISO 10993-19
In addition to these compulsory assessments further chemical characterisation could be performed to better characterise your medical devices (FTIR, SEM, DSC, SEC). More detail in the chemical characterisation.
An essential part of the biological evaluation of medical devices, this process involves a comprehensive analysis of the physical, chemical, and surface properties of the materials used in medical devices to ensure their safety, performance, and biocompatibility. Understanding these properties will help you predict how the material will interact with biological systems.
The ISO 10993-19 standard covers many different dimensions, from the analysis of the elemental and molecular composition of materials to its thermal properties, the surface structure and morphology or the surface wettability and hydrophobicity.
Degradation product analysis (ISO 10993-13/14/15)
Another crucial aspect is to control the evolution of the device over time.
Uncontrolled degradation by-products and manufacturing product is ultimately something that need to be verifying to insure long term safety of the devices both in storage conditions and in living conditions
Through this process we can understand the long-term stability and safety of polymer-based medical devices, identifying and quantifying any by-products released as the material breaks down over time.
In this part of biocompatibility, stability testing is performed (linked to the sheet) and similar tests as ISO 10993-18 are performed after aging. For metallic implant corrosion resistance and metallic leaching could be evaluated.
What Biocompatibility Services Do We Offer?
We offer a range of biocompatibility services for our clients:
Other In Vitro and In Vivo Studies
Performed outside a living organism (typically in a controlled laboratory environment), the in vitro and in vivo studies are crucial for assessing the biological response to the medical device. There are 3 different tests we can perform, according to different standards:
- ISO 10993-5 - Cytotoxicity
- ISO 10993-10 - Irritation skin sensitisation
- ISO 10993-11 - potential systemic toxicity of medical devices
What Are the Benefits of Biocompatibility Testing?
Testing the biocompatibility of medical devices is an essential service that we perform to ensure the safety and compatibility of these devices with biological systems and ultimately protect the health and well-being of patients who rely on them for medical treatment and care. Among all the benefits this testing includes, we would like to highlight:
Why Choose Applus+ Laboratories for Biocompatibility Testing?
Choosing Applus+ Laboratories for your biocompatibility assessment aligns you with a distinguished leader in medical device testing. Our services, compliant with FDA requirements, and ISO standards, are specifically crafted to guarantee the structural integrity and safety of your medical device
- Fully equipped recent lab with the latest technologies
- Strong skills expertise on material evaluation
- Recognised and certified laboratories and employees
With operations across multiple countries, Applus+ Laboratories brings our leading-edge packaging validation testing services to clients worldwide, ensuring accessibility to superior testing solutions wherever you are located.
We are a global company and thus we deliver our testing services anywhere in the world, providing top-tier biocompatibility testing to all our clients.
Trust Applus+ Laboratories as your dependable partner for biocompatibility testing. We stand ready to support your efforts with our extensive services and expert insights.